Cancer Research Catalyst offers a quarterly review of the most recent cancer therapies from the FDA to assist our readers in keeping track of those approved by the U.S. Food and Drug Administration( FDA ), comprehending their effects on patients, and placing them in the context of today’s therapeutic landscape. & nbsp,
A modified stem cell therapy based on umbilical cord blood, two PARP inhibitors for the treatment of specific prostate cancers, and antibody-drug conjugates for various cancer types were all approved between April and June 2023. & nbsp,
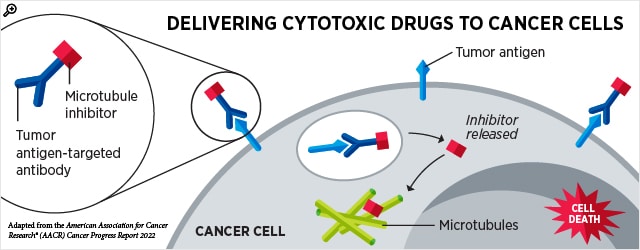
Using antibodies to target cancer cells and nbsp,
Traditional cancer therapies like chemotherapy and radiation therapy both kill cancerous and non-cancerous cells, which can cause patients to experience incapacitating side effects. Newer cancer treatments aim to selectively kill cancer cells while protecting healthy cells in order to lessen these side effects. In addition, & nbsp,
Using antibodies, which can be engineered to recognize proteins that are primarily found on cancer cells, is a strategy for targeting cancerous cells. An antibody-drug conjugate, a type of therapy, is one in which the toxic drug is connected to an antibody. The antibody-drug conjugate is internalized when the antibody binds to the cancer cell’s target protein, and the toxic drug is then released into the cell, where it kills the cells. Due to this design, the drug can only be injected into cancer cells, which are typically the cells that express the target protein. & nbsp,
The FDA has granted several antibody-drug conjugates for a number of cancer types. Two more have received FDA approval over the past three months, one for bladder cancer and the other for specific hematologic cancers. & nbsp,
- As a first-line treatment for locally advanced or metastatic bladder cancer, enfortumab vedotin – ejfv( Padcev) was approved in April in conjunction with pembrolizumib ( Keytruda ). Enfortumab vedotin – ejfv, which was first described in a 2016 report in the AACR journal Cancer Research, is made up of the cell-killing drug MMAE and an antibody that binds the protein nectin-4 found on many bladder cancer cells. & nbsp,
- Additionally, in April, polatuzumab vedotin-piiq( Polivy ) was approved as a first-line treatment for some patients with diffuse large B-cell lymphoma, not otherwise specified( DLBCL – NOS ) or high-grade B, cell lymph, ( HGBCL ). This treatment is commonly referred to as R-CHP. The CD79b protein, which is expressed on the surface of malignant B cells, is bound by poliatuzumab vedotin-piiq, and when the antibody-drug conjugate is internalized, MMAE is released into the cells and causes cell death. & nbsp,
A steroid and chemotherapy regimen that includes the chemotherapeutic vincristine( also known as R – CHOP ) is the standard first-line treatment for patients with these cancers, but only 60 % of patients experience long-lasting effects. For this patient population, the approval of polatuzumab vedotin-piiq plus R-CHP offers a more efficient treatment option. Patients who received first-line polatuzumab vedotin-piiq plus R-CHP were found in a clinical trial to be significantly less likely to experience disease progression than patients receiving standard R – CHOP therapy. & nbsp,
Enfortumab vedotin-ejfv was previously approved as a monotherapy for patients with metastatic or locally advanced bladder cancer who had already undergone treatment. The most recent approval allows for the drug’s use in patients who have not yet received treatment, many of whom are ineligible for first-line standard-of-care chemotherapy and combine it with immunotherapy. Enfortumab vedotin-ejfv induces the release of immune-stimulating molecules from dying cells, according to research presented at the AACR Annual Meeting 2020. As a result, combining the therapy with pembrolizumib may increase the immune response against the tumor. & nbsp,
Antibodies can be used to direct cytotoxic immune cells to cancer cells in addition to toxic drugs. Bispecific T cell engagers( BiTEs ) are antibodies that bind two proteins — one on T cells and the other on cancer cells — at the same time, creating a bridge that connects the two cells. In addition, & nbsp,
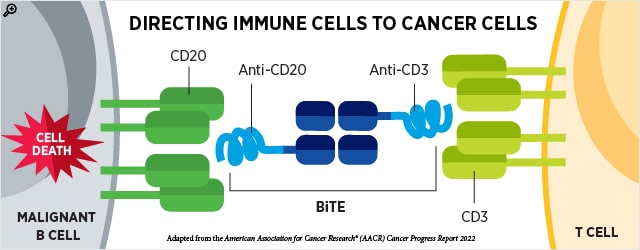
Two BiTEs were given accelerated approval by the FDA this quarter to treat patients with specific relapsed or resistant B-cell lymphomas. Accelerated approval implies that a confirmatory trial may be necessary for continued approval. & nbsp,
Patients with relapsed or resistant B-cell lymphomas have historically had few options for treatment. Another option has been made available by the development of chimeric antigen receptor ( CAR ) T-cell therapy in recent years, but many patients lack the time or resources for this highly specialized and personalized therapy. The recently approved BiTEs provide patients with specific B-cell lymphomas with efficient off-the-shelf treatments. In addition, & nbsp,
- Epcoritamab-bysp( Epkinly ) was given accelerated approval in May as a treatment for patients with relapsed or resistant DLBCL-NOS or HGBCL who had received at least two prior systemic therapy sessions. A BiTE called epicoritamab-bysp binds the CD3 receptor on T cells as well as CD20 on malignant B cells. The FDA has now approved this BiTE for DLBCL or HGBCL, making it the first of its kind to be approved for hematologic cancers. The AACR journal Cancer Discovery published earlier findings from the clinical trial evaluating epcoritamab-bysp.
- Glofitamab – gxbm ( Columvi ) was given accelerated approval in June to treat patients with relapsed or resistant DLBCL-NOS or large B-cell lymphoma ( LLBCL ) from follicular lympholi who had already undergone two or more systemic therapy sessions. Glofitamab – gxbm is a BiTE that binds the CD3 receptor on T cells and CD20 on malignant B cells, similar to epcoritamab-bysp. Features linked to clinical response to glofitamab – gxbm were discovered in a study presented at the AACR Annual Meeting 2022. & nbsp,
A BiTE that had received an accelerated approval in 2018 to treat specific leukemias was also given full FDA approval this quarter. & nbsp,
- For the treatment of adult and pediatric patients with CD19-positive B-cell precursor acute lymphoblastic leukemia ( B – ALL ) who are in first or second complete remission and have a certain level of minimal residual disease, blinatumomab( Blincyto ) received full approval in June. A BiTE called blinatumomab binds CD19 to leukemia cells and the CD3 receptor to T cells. In addition, & nbsp,
modifying stem cells derived from the umbilical cord blood, nbsp,
Hematologic malignancies may require stem cell transplantation to replace cancerous blood cells with hematopoietic stem cells, which will later mature into healthy cells. For this procedure, hematopoietic stem cells are primarily found in the blood of the umbilical cord, but their use has been linked to fatal infections that develop before the healthy blood cells have repopulated and the immune system has recovered. In addition, & nbsp,
The FDA has approved a modified strategy this quarter that involves treating umbilical cord blood with nicotinamide before transplantation to increase the number of hematopoietic stem cells. This strategy may reduce the risk of these infections. In addition, & nbsp,
- Patients 12 years of age and older who are scheduled for myeloablative conditioning and subsequent umbilical cord blood transplantation as a treatment for their cancer are eligible for the therapy, omidubicel – onlv ( Omisirge ). Omidubicel-onlv led to faster neutrophil recovery and was linked to a lower risk of some infections when compared to the conventional approach, in which umbilical cord blood is not pretreated with nicotinamide. & nbsp,
Earlier availability of PARP inhibition for patients with metastatic castration-resistant prostate cancer
Patients with metastatic castration-resistant prostate cancer ( mCRPC ) typically receive chemotherapy or androgen-blocking therapy, but the majority of patients only live for three years after receiving these therapies. Inhibitors of the PARP protein, which controls androgen receptor activity and is involved in DNA repair, may be used to treat some patients whose mCRPC progresses on andromen-blocking therapy. In addition, & nbsp,
Due to two FDA approvals this quarter, PARP inhibition was made available to patients along with androgen-blocking therapy, relegating it to an earlier course of treatment. In addition, & nbsp,
- For patients whose mCRPC harbors BRCA gene mutations, the FDA approved olaparib ( Lynparza ), abiraterone( Zytiga ), and corticosteroid in May. & nbsp,
- For patients whose mCRPC harbors mutations in a BRCA gene or in other genes involved in homologous recombination, an act of DNA repair, the FDA approved talazoparib ( Talzenna ) in June along with enzalutamide ( Xtandi ). & nbsp,
Olaparib and talazoparib have been given approval to treat a number of BRCA-mutated cancers, which are thought to accumulate DNA damage and ultimately result in cell death due to the combined suppression of the DNA repair processes that involve both PARP. Olaparib and rucaparib( Rubraca ), two PARP inhibitors, were approved by the FDA in 2020 to treat BRCA-mutated mCRPC that develops after receiving androgen-blocking therapy like abiraterone or enzalutamide. In addition, & nbsp,
The most recent approvals are based on clinical trial data demonstrating that patients with mCRPC harboring specific genetic mutations had a lower risk of disease progression or death when given olaparib, talazoparid, or enzalutamide, respectively. In addition, & nbsp,
Visit the FDA approvals page on the AACR website for more information, including the complete indications and the clinical trial outcomes that resulted in each approval. & nbsp,
The American Association for Cancer Research ( AACR ) first published the post FDA Approvals in Oncology: April – June 2023.