Tumors arising in the gallbladder, bile ducts, and the small intestine are collectively referred to as biliary tract cancers. These cancers are rare—affecting approximately 12,350 individuals in 2024—but they are typically diagnosed at advanced stages when surgery, which can improve prognosis, is no longer an option.

Systemic therapies, therefore, are the mainstay of treatment for most patients with this disease. Chemotherapy regimens have long been approved to treat advanced biliary tract cancers, and in recent years, immunotherapy and targeted therapy have joined the treatment landscape.
Despite these advances, many patients’ biliary tract cancers eventually return, underscoring the need for more effective strategies. A recent commentary published in the AACR journal Clinical Cancer Research by researchers Angela Lamarca, MD, PhD, from Hospital Universitario Fundación Jiménez Díaz in Madrid, Spain, and Teresa Macarulla, MD, PhD, from Vall d’Hebron Institute of Oncology in Barcelona, Spain, highlighted how current treatments tend to approach this heterogeneous group of cancers as a monolith and suggested ways to make treatment more targeted to each type of biliary tract cancer or to each patient.
New Treatment for Biliary Tract Cancer Targets HER2
One approach they suggested is to develop therapeutics against more targets since matching patients with therapies that target the molecular features driving their biliary tract tumor may lead to better responses. The U.S. Food and Drug Administration (FDA) has in recent years approved several therapies for biliary tract cancers that target some of these molecular features—pemigatinib (Pemazyre) and futibatinib (Lytgobi), both of which inhibit FGFR2, and ivosidenib (Tibsovo), which inhibits IDH1.
But, as Lamarca and Macarulla noted, it has become increasingly evident that different types of biliary tract cancers harbor different molecular features: FGFR2 and IDH1 mutations, for example, are frequently found in bile duct cancers that start in the liver (the most prevalent type of biliary tract cancer) but not in bile duct cancers outside the liver nor in gallbladder cancers.

This means the approved FGFR2 and IDH1 inhibitors may not be as effective in the less prevalent biliary tract cancers driven by other alterations. The authors therefore argued that advancing drugs against the alterations common in these other types of biliary tract cancers should be a priority. One promising therapeutic candidate the authors proposed was HER2, a protein found at high levels in many of the cancers that form in the gallbladder and in the bile ducts outside the liver.
The authors summarized some recent clinical data supporting the efficacy of investigational HER2-targeted therapies, and in a major advance for biliary tract cancer treatment, the FDA has since approved the first HER2-targeted therapy for these cancers. The drug, zanidatamab (Ziihera), binds to HER2 in two different places, a novel design that is predicted to make it more effective than other HER2-directed therapeutics that only bind one site. It was approved in November 2024 to treat previously treated, advanced biliary tract cancers that harbor HER2 mutations.
Novel Biomarkers to Predict Immunotherapy Response
Lamarca and Macarulla also highlighted the need for new biomarkers to improve precision for immunotherapy. Although the approval of immune checkpoint inhibitors has transformed the standard of care for biliary tract cancers, most patients don’t benefit, pointing to the need for novel biomarkers that better predict whose tumors will respond to this form of immunotherapy. Doing so could spare patients the toxicities and costs associated with the treatment if they are unlikely to have a response, and it would allow them to consider other treatment options that might be more successful.
While some biomarkers are commonly used for this purpose in other cancer types, they have been largely unreliable for biliary tract cancers. Fortunately, researchers continue to explore new avenues, with one innovative approach recently reported in Clinical Cancer Research.
In the study, researchers examined whether the distribution of immune cells in and around a patient’s tumor could offer clues about their chances of response. Because analyzing tumor tissue can be tedious and subjective, the researchers used an artificial intelligence (AI)-based model that could report immune cell distribution based on digital images of the patient’s tumor tissue.
The model categorized biliary tract tumors from 339 patients into one of three categories: inflamed (high density of immune cells), immune-excluded (lower density of immune cells), or immune desert (lowest density of immune cells). In a retrospective analysis, these categories were significantly associated with response to immunotherapy, with responses in 27.5% of patients with inflamed tumors compared with 7.7% of those with either immune-excluded or immune desert tumors. Patients with inflamed tumors also lived longer—a median of 12.6 months compared with 5.1 months among those in the other categories.
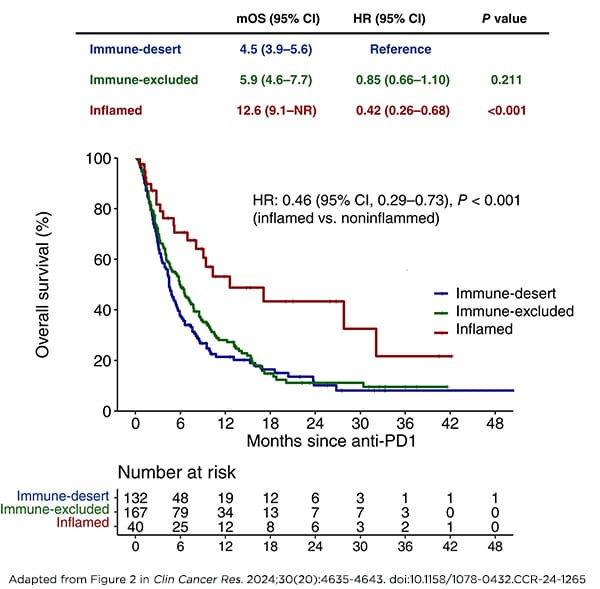
Therefore, the researchers suggest that immune cell density could be a valuable biomarker to more precisely select patients for immune checkpoint inhibition, particularly given the emergence of this treatment class as the standard of care for advanced biliary tract cancers.
As research continues to drive advances against this rare but deadly group of cancers, efforts to make treatment more tailored will be key to improving survival. This will require a deeper understanding of the molecular differences across biliary tract tumors, reliable biomarkers to ensure patients receive the treatment best suited to their individual cancer, and insights into resistance mechanisms and strategies to overcome them.
The post Making Biliary Tract Cancer Treatment More Precise appeared first on American Association for Cancer Research (AACR).

