The most recent paper that asserts they can do it is presented here. It appeared in the journal Science Translational Medicine. This journal is run by Science and is fairly reputable. This research team even established a new biotech company to commercialize their strategy because they are so certain of their data. I’m going to look at how credible their claims are.
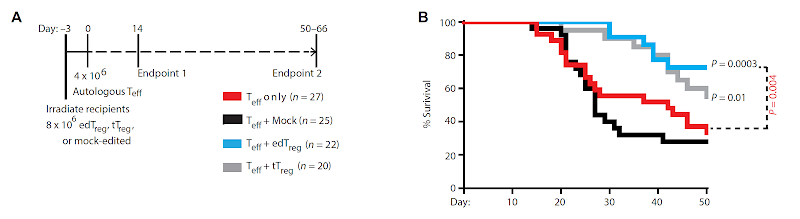
However, there are some inconsistency between experiments describing GvHD model. In one set of experiments it produced 100 % lethality by day 21( see below, red line) while in other set of experiments it produced only 20 % lethality( see above, red line ). & nbsp,
 |
Such inconsistency casts doubts about edTregs ability to inhibit effector T cells in vivo and could explain why the authors did not see much difference in GvHD scores with or without edTregs( see below ).
 |
|
& nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp, & nbsp,
This could also explain why the authors did not see improvement in brain inflammation in mice EAE model when co – transferring antigen – specific edTregs with effector T cells.
|